Abstract
Immune system mediates the tumor progression as the protective actions of the immune cells are suppressed, and they assist the tumor to grow by various mechanisms. This review illustrates the mechanisms adapted by the T regulatory cells, T helper cells and B lymphocytes that result in decreased immunosurveillance and poor tumor progression. Clear understanding of the pathways adopted, and mediators released by the immune cells can elaborate various target sites to explore in the cancer treatment. Stimulating immune response and recognizing cancer cells as non-self-antigens can be an optimum approach to fight tumor.
Key Words
Tumor, Immune system, T-helper cells, T-regulatory cells, Immune suppression
Introduction
Human mature B cell and T cell malignancies justify research into their molecular circuitry and pathogenesis. This review comprises of the pathogenesis of cancer mediated by the immune cells T and B cells more specifically. Two key classes of CD4+ Tregs with distinct roots are thought to exist: n Tregs and i Tregs (Normal T regs and adaptive or induced T regs). In the thymus, nTregs evolve and are initially categorized as CD4+ T cells and Fox P3 transcription factor is expressed by them. Moreover, traditional naïve T cells (CD4+Fox P3-) can also be converted to CD4+ Fox P3+ T cells producing so called iTregs (Elkord et al., 2010). The newly renowned T-Helper (Th17) cells, which are recognized in the field of cancer studies and play an important part in Tumorigenesis. Th17 cells significantly release IL-17 in TME which is a proinflammatory cytokine. Other cell types have also been shown to generate the cytokine (Ernst & Putoczki, 2014).
The profiling of gene expression and other high-resolution molecular technologies have shown that DLBCL is genetically heterogeneous. Most cases can be split into one of two main molecular subtypes according to gene expression profiling, a subtype of germinal core B cells according to their origin cell or it can be a B cell that is activated (Cunningham et al., 2013).
T-Regulatory Cells
Natural and induced T reg cells are two sorts of T regs based on their origin. Natural Tregs originate from Thymus and migrate from there to periphery to accomplish their task of Immune Homeostasis. These natural Tregs get their suppressive ability from the specific microenvironment upon recognition of self-antigens presented on stromal cells (Huber et al., 2004; Voron et al., 2014). Treg development inside the thymus involves the stimulation of TCR and is dependent upon Co-stimulation of B-7 (Ghiringhelli et al., 2005; Jordan et al., 2001).
Natural Tregs express PD-1, CD25, CCR4, Foxp3 and CD127 and their suppression mechanism is related with the secretion of various cytokines including IL 10 & IL 35. These Tregs adopt a specific T-cell lineage derived through thymus permitting them to suppress the activation of APC’s i.e., B cells, monocytes, and DC’s (Dendritic cells). T regulatory cells are reliant on Interleukin-2 for their proliferation and show high levels of CD25. However, transcription factor FoxP3 is the utmost exclusive marker specific to T regulatory cells responsible for the immunosuppressive function of FoxP3+CD25+ Tregs (N. Trehanpati & Vyas, 2017).
Induced Tregs do not develop in thymus but outside it under various physiological conditions including stress and disease conditions. They are induced by the disease process and inflammation e.g., cancer.
Although nTregs and iTregs have similar functions but have some epigenetic differences like former has more stable FoxP3 expression and methylation.
Tregs Associated Immunosuppression and favoring Tumor Progression
The T regulatory cells levels rise significantly in peripheral blood in Lungs, ovarian and gastric cancers (M. H. Chang, 2000; Huber et al., 2004). Their number is almost double in cancer patients as compared to normal individuals (Asano, Watanabe, Kitani, Fuss, & Strober, 2008; Zheng, Wang, Wang, Gray, & Horwitz, 2007). Various studies have demonstrated the tumorigenesis and survival of cancer patients associated with large amount of these regulatory cells in the TME (L. W. Collison et al., 2007; Gavin et al., 2007; Nirupama Trehanpati et al., 2012). FoxP3+ Tregs play the lead part in the Immunosuppression and tumor progression via different mechanisms and lead to poor clinical outcomes by the development of the resistance to antitumor therapies e.g. immune checkpoint inhibitors (Franzese et al., 2005; Jordan et al., 2001; Stoop, van der Molen, Kuipers, Kusters, & Janssen, 2007; Su et al., 2013; Yu et al., 2014).
Entry of Tregs into TME (Tumor Microenvironment)
Various Chemokine receptors expressed on Tregs
are responsible for the chemotactic recruitment of the Tregs to the TME (39,50). Chemokines i.e. CCL5, CCL6, CCL17, CCL22 and CCL28 released by those immune cells that are associated with the tumor as well as some tumor cells, attract the activated T regulatory cells that express the CCR4, CCR5, CCR10 and CXCR3 receptors from peripheries towards the microenvironment around tumor (M. H. Chang, 2000; Jordan et al., 2001; Karimi-Googheri et al., 2014). CCL-22 (chemokine ligand-22) secreted by tumor cells and macrophages in levels in Hodgkin Lymphoma (Ishida et al., 2006) ovarian (Curiel et al., 2004) and recruit CCR4+Tregs to the TME. Dendritic cells residing in TME and tumor cells produce high levels of Interleukin 10 (Seo, Hayakawa, Takigawa, & Tokura, 2001), TGF-? (Liu et al., 2007) along with Adenosine (Zarek et al., 2008) that promotes the expansion and generation of Tregs in the TME.
Tregs in the Tumor Microenvironment mediate various cellular pathways and mechanisms reduce the immune function and cause tumor growth.
Mechanisms of Immune Suppression by Tregs
Mechanisms by which Tregs play their immunosuppressive role include the contact dependent & Independent mechanisms (Vignali, Collison, & Workman, 2008). Apoptosis of the neighboring T-cells due to the high consumption of Interleukin-2, Immunosuppression through CTLA-4 and Foxp3 signaling pathway, Secretion of perforins & granzyme B to kill the DCs are contact dependent. whereas the production of cytokines and those mediators that are immunosuppressive in nature (Strauss et al., 2007) decreasing response constitute one of the contact independent mechanisms. Interleukin-35 has been associated in a study with the optimum suppressive activity of Tregs due to high expression on these cells (L. W. Collison et al., 2007). Furthermore Tregs & DCs interactions mediated through the CTLA4 leads to upregulation of IDO gene (Indolamine 2,3 - dioxygenase) in DCs which sequentially increases the Tryptophan breakdown and stimulating the destruction of T effector cells over the depletion of Tryptophan. Increase in the extracellular
Adenosine by the tumor cells probably due to hypoxic environment promotes the tumor progression and immunosuppression (N. Trehanpati & Vyas, 2017).
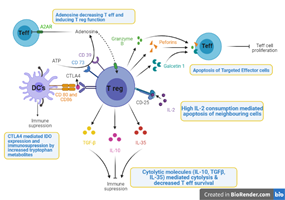
Figure 1
Mechanisms of Immunosuppression in TME by the T regulatory Cells through the Apoptosis of T effector cells by IL 2 Deficiency as well as the apoptosis mediated through Granzyme B, Perforins and Galcetin 1, Cytokines mediated Cytolysis of T effector cells, Decreased immune response by CTLA4 mediated increase in the tryptophan metabolism and role of adenosine to induce the T regulatory cells function (Created with Biorender.com)
Apoptosis of T Effector cells Mediated by IL-2 Deprivation
Within the TME Tregs and Teffs compete for the Interleukin 2 (Busse et al., 2010). Expansion of T regulatory cells along with their immunosuppressive function is regulated by IL-2 (Jin et al., 2014; Thornton et al., 2019; Turnis et al., 2016). Moreover, the Survival and Proliferation of Teffs (Malek, 2008) and Tregs (Cheng, Yu, & Malek, 2011) is dependent upon IL-2 Signaling. Increased levels of Interleukin 2 receptor ?-chain i.e. CD-25 on surface of Tregs have increased binding affinity to the IL-2 (Cheng et al., 2011; Chinen et al., 2016) hence Tregs act as sink for the IL-2 consumption and end up promoting their own proliferation and survival and in turn deprives the T effector cells & suppress their role of Tumor suppression (Cheng et al., 2011; Chinen et al., 2016; Thornton et al., 2019).
Cytolytic Molecules (TGF-?, IL-10 and IL-35) mediated Cytolysis
Il-10 & IL-35 (Xue, Yan, & Kan, 2019) has been reported in various studies to be significant for the immunosuppression activity and promoting Tumor progression. Interleukin-10 has been shown to cause the inhibition of DCs Maturation, decreased production of granzyme B and favoring tumorigenesis, whereas decreased levels of IL-2 has been related with the inhibition of Treg function and increased T effector cells cytotoxic function reducing the Tumor growth (S. Wang et al., 2016). Interleukin 35 produced by Tregs has shown to decrease the survival and proliferation of Teffs (Turnis et al., 2016) through the cell cycle arrest induced by the upregulation of immune checkpoints present on the T effector cells (Pylayeva-Gupta, 2016).
TGF-? is also a significant mediator for the proliferation and immunosuppressive activity of the Tregs (Bommireddy & Doetschman, 2007; Seoane & Gomis, 2017; Strauss et al., 2007), they favor the Treg recruitment in the TME and increase the Treg mediated deprivation of Teffs and cytolysis (Cao et al., 2007; Dedobbeleer, Stockis, van der Woning, Coulie, & Lucas, 2017; Stockis et al., 2009).
CTLA4 Mediated Immunosuppression
T regulatory cells express the receptor CTLA-4 (Cytotoxic T lymphocyte antigen-4) in high levels which binds favorably with CD80 & 86 present on the DCs and this binding stimulates the catabolism of Tryptophan inside the DCs through the increased IDO gene expression, causing the increased Tryptophan metabolism and Increased immunosuppressive Tryptophan metabolites which in turn leads to the suppression of the
function of the Teffs.
Furthermore, inhibition of DCs development and death of DCs via IL-10/ TGF-? mediated signaling pathway and perforin dependent pathway respectively decreased the DCs mediated CD8+ T cell activation and decreased immunosurveillance.
Targeted Cytolysis by Tregs
Another contact dependent mechanism mediating the immunosuppressive role of the Tregs is the targeted killing of Teffs by the chemicals secreted by Tregs. Activated Tregs in the tumor environment secrete high levels of suppressive molecules i.e., perforin, granzyme B and Galcetin-1 that promotes the cytolysis of the targeted effector cells by triggering apoptosis. This precisely kills the B cells by granzyme dependent pathway and effector cells by the TRAIL-DR5 signaling pathway (Zhao, Thornton, DiPaolo, & Shevach, 2006).
Adenosine Mediated Immunosuppressive Function of Tregs
ATP inside the Tregs is catalyzed to the Adenosine which has been shown to decrease the functions of the Teffs (Vignali et al., 2008). Adenosine mediated suppressive pathways (Borsellino et al., 2007) by the FoxP3 expressed Tregs showing increased levels of CD73 & CD39 that inhibit the T effector cells proliferation ( Deaglio et al., 2007; Sitkovsky et al., 2008) along with Immunosurveillance, impairs the NK cells Activation, inhibit the Antigen presenting cell’s function and stimulates the Tregs Function (Ohta & Sitkovsky, 2014).
T-Helper Cells
They are CD4 cells the subtype of T cell responsible for the defense system of human. Immunity provided by this is typically the acquired immunity which on the contrary to innate immunity a person develops when its immune system encounters certain pathogens or foreign agents. normally it plays a beneficial role to humans helping the person's immune system develop protection but, in some cases, its secretions, reactions, byproducts can also harm humans by causing autoimmune diseases and helping in the pathogenesis of cancer.
After decades of research in this field we have divided the helper t cells into 5 subsets according to products
T helper cells Mediated Cancer Progression
Cancer can be caused due to genetic reasons, viruses and sometimes even bacterial infections, environmental carcinogens, and radiations (Blackadar, 2016; Bodmer, 1994; Clapp, Jacobs, & Loechler, 2008; Hussain, Hofseth, & Harris, 2003)
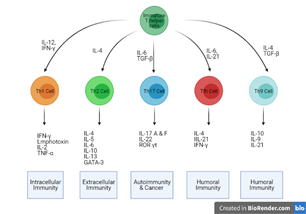
Figure 2
Development of the Naive T cell into a particular group of T helper cells (Created with Biorender.com)
Genetic reasons involve mutations in genes and two types of genes play central role in this i.e., oncogenes and tumor suppresser genes.
Experiment was performed on mice and they found that ROR? released by T helper cells is a modulator of immune stability and can prove to be an excellent target in inflammatory diseases like cancer (Ivanov et al., 2006).
T helper 17 cells produce tumor vasculature especially in immune lacking mice it increases the level of IL6 which also regulates IL17 level and activates the STAT 3 pathway lead to the formation of oncogenic factors that increase proangiogenic factors which ultimately promotes angiogenesis (Chehimi, Vidal, & Eljaafari, 2017).
IL 17, secreted by TH17 cells, is a pleiotropic proinflammatory cytokine, which can further increase cancer evoked inflammation since th17 cells are the major source of IL 17 some other types of cells also secrete the IL17 when they encounter the specific stimulus whether it is in vitro or in vivo for example Natural Killer cells. (Song & Yang, 2017).
IL 17 is a cytokine family in which certain subtypes of IL 17 coordinates the activation of certain T cells and immune responses generated by them. Various studies have been performed and results have shown that IL 17 mediates the pathogenesis of autoimmune diseases, malignancy, allograft transplantation, and angiogenesis. The levels of IL 17 cytokines are remarkably increased in different types of tumors (Alinejad, Dolati, Motallebnezhad, & Yousefi, 2017; Coffelt et al., 2015), and gastric cancer. Various studies have been performed and it was indicated that IL 17 opens the cascade of reactions by activating various channels which may lead to further progression of cancer.
Capability of the cancer cells to metastasize their existence is main reason of the patient’s death. A significant interaction between abnormal cancer cells and localized healthy cells and due to these interactions, there a chain of reactions startup which cause the cancer to metastasize to normal cells. A study was performed by Seth B. Coffelt, and his colleagues and it was found that the T cells and IL 17 play the astonishing role in breast cancer metastasis as such the process occurs that the IL 17 expression from gamma delta cells further cause (G-CSF) to activate which in response activate tumor induced neutrophils and they have ability to suppress the cytotoxic T cells (CD8 cells). It was concluded that the absence or downregulation of gamma delta cells can be a perfect pathway to put a stop to the metastatic disease so it cannot become so deadly (Coffelt et al., 2015). Breast cancer mainly caused by the T helper17 cells typically via the proinflammatory cytokine which is IL17 which open up the chain of reactions and produce mediators like NF-kappa B and other inflammatory cytokines through multiple channels (Alinejad et al., 2017). Another study was performed, and they found that IL 17 cause angiogenesis by activating STAT3/GIV pathway in NSCLC, For this experiment they prepared NSCLC cell lines and prepare for two scenarios i.e. one having recombinant IL 17 and other one having not and observed the effect in both. They noted that one with IL 17 treated have showed increased phosphorylation of STAT 3 then the other one which was then confirmed by immunoflouro- assays. They also found that GIV being the protein is the direct target of STAT 3, a transcription factor. They concluded that IL 17 treated cells showed increased expression of Stat 3 and GIV on the contrary when they decreased the amount of endogenous IL 17, expression both STAT 3 and GIV were decreased exponentially so it is not false to comment that IL 17 cause the angiogenesis by activating STAT3/GIV pathway (Pan et al., 2015).
Another study was performed by Xiaoqin Wu and his fellow scientists to check the effect of IL17 in Gastric Cancer. They found that IL17 being a positive stimulator of proangiogenic factors and VEGF is proved to be very troublesome in cancer responsible for angiogenesis by activation of Stat3 and GIV. Angiogenesis directly relates to metastasis so increased abnormal stat3 activation proved to be more fatal than fruitful which is being activated by TH17 cells (v). Furthermore, IL 17 on activation increase the resettling of HepG2 cells also cause the increase phosphorylation of STAT3, RT-qPCR side by side activate the IL 6 and increase its levels, the IL 6 further cause phosphorylation of STAT3 so IL17, IL6, STAT3 pathway is one of the efficient and beneficial targets for Hepatocellular carcinoma (HC) caused by hepatitis B virus (Hu et al., 2017). Almost same type of work done by Lin Wang and his colleagues but they used mice in their experiment but reached to the same conclusion that IL 17 can result in increased tumor growth through activation of IL6, STAT 3 pathway (L. Wang et al., 2009).
A study was performed to check the effect and levels of IL17, IL22, and IL23 on colorectal cancer patients having K-ras mutation. Colorectal cancer is cancer in which the mutation occurs at the genetic level either on the oncogenes or tumor suppressor genes. Their level is disturbed either overexpressed or under-expressed. The PIK3CA gene is overexpressed while the tensin homolog and tumor suppressor gene is under-expressed. To find about the reasons and reactions which promote carcinogenesis, a biomarker K ras is used because of its history that it is a positive activator of Stat 3, PIK3 and MAPK. This marker mediates cell division, cell differentiation and angiogenesis and thus present in relatively high amount in almost all type of cancers involving colorectal carcinoma, adenocarcinoma, and lungs cancer (Petanidis, Anestakis, Argyraki, Hadzopoulou-Cladaras, & Salifoglou, 2013).
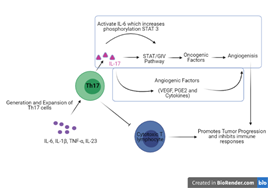
Figure 3
Mechanisms of Decreased immunosuppression promoting the tumor progression by the IL-17 secreted by the T Helper cells (Created with Biorender.com)
IL17 exert a protumor effect by stopping Cytotoxic T lymphocytes activities and inducing myeloid-derived suppressor cells tumor progression. Th17 cells act as a double sword, it recruits the macrophages in defense of body but also its mediator which is mainly IL17 produce proinflammatory cytokines like NF Kappa B promotes cancer by recruiting more myeloid-derived suppressor cells and the result is tumor progression at exponential rate (Hemdan, 2013). Th 17 cells induce the transcription factors ROR?t/RORC2 and ROR? which produce IL17 and other proinflammatory cytokines and involve in causing different types of lung tumor (Marshall et al., 2016) .Th17 cells also play the pathogenic role in lung cancer. A study was performed by Seon Hee Chang and his co scientists in which they used mouse model which have activated K-ras gene, the levels of Th17 and T regulatory were found prominent in comparison to Th1 cells. After that mouse were regularly given nontypeable Haemophilus influenza (NTHi) for a week the results showed that there is a high level of tumor growth and after that they removed the supply of IL 17 and to their surprise the tumor growth was controlled even in those who are treated with NTHi which is a tumor growth promotor. They concluded that IL 17 is the major cytokine around which all the story of cancer is pivoted Th17 cells itself not have such magnificent effect but its cytokine is the major cause of cancer (S. H. Chang et al., 2014).
Pathogenesis of Cancer by B cells
The antitumor role played by B cells through various ways includes the production of antibodies, cytokines, and antigen-antibody complexes formation. By autoantibodies and tumor growth factors production the B-cells may facilitate the formation of tumors. The Regulatory B cells can suppress the responses of T Helper1 and CD8+ cytolytic T cell directly and indirectly.
B cells Development
They are formed in the bone marrow from
hematopoietic stem cells. Multipotent progenitors are produced from hematopoietic stem cells produce that are characterized by response of identified developmental markers into common lymphoid progenitor. Some cells are associated with the B-line by expressing transcription factors and cytokine receptors as well as by the bone marrow environment (Ademokun, Wu, & Dunn-Walters, 2010).
Antibodies are mainly produced by the B-lymphocytes in the immune system. From progenitor B cells to mature circulating B cells development process there is a membrane-bound heavy chain which plays a key role by becoming a part of the preBCR and BCR (Reth & Nielsen, 2014). Normal differentiation and activation of cells B are largely overturned to their boundless development and survival by B-cell lymphomas. The machinery for diversification of antibodies may induce chromosomal translocation and oncogenic mutations, as B cells are particularly vulnerable to malignancy (Shaffer III, Young, & Staudt, 2012).
Malignant Development of B cells
As the B cells mature they pass on from various stages that include Pro B cells, Pre B cells and then immature B cells to translational and Mature B cells based on the ability to produce certain markers and antibodies (Loder et al., 1999). For expression of suitable surface receptor B cell growth, i.e. pre-B cell receptor at stage pre-B, and Surface immunoglobulin (Ig) at the transformation from pre-B cell to mature B-cell, B lymphocytes are frequently selected (R. Küppers, Zhao, Hansmann, & Rajewsky, 1993).
B Cells in Cancer
B cells represent almost 40 percent of lymphocytes in TME of breast malignancy and 25 percent of all the tumor cells approximately (Coronella-Wood & Hersh, 2003). Consumption of CD20-expressing B cells expanded tumor load in mice lung infused with B16-F10 melanoma (Sorrentino et al., 2011). The transformation and amassing of B cells and the antibodies released by those cells may assume definitive jobs in the formation of tumor. The reduced antitumor immune response was observed due to the presence of B cells in a limited stockpile of CD4+ T cells (Shah et al., 2005).
Human B cell Lymphoma cellular origin
The microarrays to produce the methods for the expression of genome-wide gene were far more detailed than was commonly achievable for single or several marks through immunohistochemical staining when comparing the normal B cells subsets and the gene expression of B cell lymphomas of human. Consequently, such similarities have been commonly used to describe the cellular origin of lymphomas in recent years (Alizadeh et al., 2000; Rosenwald et al., 2002).
The heterogeneous diffuse group of B-cell lymphoma could be separated into many sub-groups. The most prevalent form is DLBCL, accounting for around 33% of all NHL cases. Two major subgroups have shown a high relation between lymphoma cells and GCB-DLBCL or B in vitro activated cells. The GCB-DLBCL has also demonstrated continuous somatic hypermutation, and it is known now as another GCB cell lymphoma. The ABC-DLBCL phenotype is strongly activated and has V-genes with somatic mutation, but most essential characteristics are absent of GC B cells. That lymphoma is closely linked to the post GC immune blood cells (Lossos et al., 2000).
The classical Hodgkin lymphoma is a special case as the, tumor cells of this malignancy, show only a few B cell markers and several markers of different types of hematopoietic cells (Ralf Küppers, 2009). In one-third of the cases, unexpectedly, disruptive somatic mutations detected and, on this observation, it has been hypothesized that those HRS cells come from a pre-apoptotic pool of the GC B cells with adverse mutations which have usually undergone apoptosis (Kanzler, Küppers, Hansmann, & Rajewsky, 1996; R Küppers et al., 1994).
B cells live in a GC for a few days to weeks only, and novice B cells constitute almost 50% of the B cell pool. Robust Proliferation can be an important factor of GC cells (Seifert, Scholtysik, & Küppers, 2013).
Targeting of B Cell Lymphoma
Rituximab is given with the CHOP- based therapy is the current first-line treatment for DLBCL (Tilly et al., 2015).
It is possible to divide BCR, a membrane-bound immunoglobulin, into five groups (Immunoglobulin A, D, E, G & M) are essential for the identification of antigens and the starting of signaling events leading to survival, migration of B cells, and proliferation (Venkitaraman, Williams, Dariavach, & Neuberger, 1991). CD79a (or Immunoglobulin A) and CD79b (or Immunoglobulin B) complex formed by BCR both contain a motif of activation-dependent on immunoreceptor tyrosine (ITAM) for transmitting signaling intracellularly.
BCR signaling is disrupted in most of the lymphomas derived from GC, resulting in enhanced proliferation of the B cells (Davis et al., 2010). GCB-DLBCL cells, by comparison, have not been destroyed, suggesting that they can survive without BCR signaling. In most of the ABC-DLBCL cases, self-antigens can contribute to persistent BCR stimulation in the tumor microenvironment (Young et al., 2015).
A novel treatment for B-NHL is currently being investigated in various Small Molecule Inhibitors (Syk, Lyn, PI3K, BTK) targeting signaling proteins under BCR. (Rickert, 2013). Nonetheless, increased recognition of the conformational modifications in the BCR on antigen-binding could make new possibilities to specifically attack the BCR itself. In the 1980s this concept was first explored, when positive therapeutic data was discovered for patients with anti-idiotype antibodies to cure their lymphoma by targeting the variable region of the BCR (Meeker et al., 1985).
Conclusion
Cancer being the world’s leading cause of death is a biggest challenge to the researchers, because the available treatments i.e. Chemotherapy, radiotherapy & immunoglobulins are not much effective in eradicating this disease and most of the treatment ends up weakening the immune system, therefore quest for new drugs and strategies having less side effects and more efficiency is always a priority for the scientists. Immune system is one of the culprits behind cancer because apart from its function i.e. protection of the body against pathogens, it is not only suppressed in cancer but favor the tumor growth. Our T regulatory cells infiltrate the Tumor Microenvironment and then by various contact dependent and independent methods decrease the immunosurveillance and suppress the immune response. Various pathways are involved in this including CTLA4 mediated killing of T effector cells, adenosine mediated decreased T effector cells proliferation, cytokines mediated cytolysis along with deprivation of T eff cells by high Inter Leukin-2 Consumption.
On the contrary, the suppression of the immune system started by T regulatory cells is further optimized by the T helper cells, by secreting IL-17 which by Stat 3, Map Kinase pathway release proangiogenic factors which cause angiogenesis. B cells further acclimatize to the TME by secreting autoantibodies, cytokines, and tumor growth factors which also suppress cytolytic T cells responses. Production of Pre-B cells and Transitional B cells having rearranged surface markers and receptors which interferes with the normal function of B cells and ultimately exaggerate the situation. Consequently, we can deduce that cancer cells camouflage the body’s defense system and make it use by their own way to grow and metastasize. We need to devise some strategy that does not eliminate the immune responses of the body towards cancer, but only those that favour tumorigenesis. In this way we can stimulate our immune system cells to fight the cancer instead of favoring it.
References
- Ademokun, A., Wu, Y. C., & Dunn-Walters, D. (2009). The ageing B cell population: Composition and function. Biogerontology, 11(2), 125-137.
- Alinejad, V., Dolati, S., Motallebnezhad, M., & Yousefi, M. (2017). The role of IL17B- IL17RB signaling pathway in breast cancer. Biomedicine & Pharmacotherapy, 88, 795-803. doi:
- Alizadeh, A. A., Eisen, M. B., Davis, R. E., Ma, C., Lossos, I. S., Rosenwald, A., Boldrick, J. C., Sabet, H., Tran, T., Yu, X., Powell, J. I., Yang, L., Marti, G. E., Moore, T., Hudson, J., Lu, L., Lewis, D. B., Tibshirani, R., Sherlock, G., . . . Staudt, L. M. (2000). Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature, 403(6769), 503-511.
- Asano, N., Watanabe, T., Kitani, A., Fuss, I. J., & Strober, W. (2008). Notch1 Signaling and Regulatory T Cell Function. The Journal of Immunology, 180(5), 2796-2804.
- Blackadar, C. B. (2016). Historical review of the causes of cancer. World Journal of Clinical Oncology, 7(1), 54.
- Bodmer, W. F. (1994). Cancer genetics. British Medical Bulletin, 50(3), 517-526.
- Bommireddy, R., & Doetschman, T. (2007). TGFβ1 and Treg cells: alliance for tolerance. Trends in Molecular Medicine, 13(11), 492-501. doi:
- Borsellino, G., Kleinewietfeld, M., Di Mitri, D., Sternjak, A., Diamantini, A., Giometto, R., . . . Falk, K. (2007). Expression of ectonucleotidase CD39 by Foxp3 Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood, 110(4), 1225- 1232. doi:
- Busse, D., de la Rosa, M., Hobiger, K., Thurley, K., Flossdorf, M., Scheffold, A., & Höfer, T. (2010). Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proceedings of the National Academy of Sciences, 107(7), 3058-3063.
- Cao, X., Cai, S. F., Fehniger, T. A., Song, J., Collins, L. I., Piwnica-Worms, D. R., & Ley, T. J. (2007). Granzyme B and Perforin Are Important for Regulatory T Cell- Mediated Suppression of Tumor Clearance. Immunity, 27(4), 635-646. doi:
- Chang, M. H. (2000). Natural history of hepatitis B virus infection in children. Journal of Gastroenterology and Hepatology, 15(5 (Suppl.)), E16-E19.
- Chang, S. H., Mirabolfathinejad, S. G., Katta, H., Cumpian, A. M., Gong, L., Caetano, M. S., Moghaddam, S. J., & Dong, C. (2014). T helper 17 cells play a critical pathogenic role in lung cancer. Proceedings of the National Academy of Sciences, 111(15), 5664-5669.
- Chehimi, M., Vidal, H., & Eljaafari, A. (2017). Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. Journal of Clinical Medicine, 6(7), 68.
- Cheng, G., Yu, A., & Malek, T. R. (2011). T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. 241(1), 63-76. doi:
- Chinen, T., Kannan, A. K., Levine, A. G., Fan, X., Klein, U., Zheng, Y., Gasteiger, G., Feng, Y., Fontenot, J. D., & Rudensky, A. Y. (2016). An essential role for the IL-2 receptor in Treg cell function. Nature Immunology, 17(11), 1322-1333.
- Clapp, R. W., Jacobs, M. M., & Loechler, E. L. (2008). Environmental and Occupational Causes of Cancer: New Evidence 2005- 2007. Reviews on Environmental Health, 23(1), 1-38.
- Coffelt, S. B., Kersten, K., Doornebal, C. W., Weiden, J., Vrijland, K., Hau, C. S., Verstegen, N. J. M., Ciampricotti, M., Hawinkels, L. J. A. C., Jonkers, J., & de Visser, K. E. (2015). IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345-348.
- Collison, L. W., Chaturvedi, V., Henderson, A. L., Giacomin, P. R., Guy, C., Bankoti, J., Finkelstein, D., Forbes, K., Workman, C. J., Brown, S. A., Rehg, J. E., Jones, M. L., Ni, H. T., Artis, D., Turk, M. J., & Vignali, D. A. A. (2010). IL-35-mediated induction of a potent regulatory T cell population. Nature Immunology, 11(12), 1093-1101.
- Collison, L. W., Workman, C. J., Kuo, T. T., Boyd, K., Wang, Y., Vignali, K. M., Cross, R., Sehy, D., Blumberg, R. S., & Vignali, D. A. A. (2007). The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature, 450(7169), 566-569.
- Coronella-Wood, J. A., & Hersh, E. M. (2003). Naturally occurring B-cell responses to breast cancer. Cancer Immunology, Immunotherapy, 52(12), 715-738.
- Cunningham, D., Hawkes, E. A., Jack, A., Qian, W., Smith, P., Mouncey, P., Pocock, C., Ardeshna, K. M., Radford, J. A., McMillan, A., Davies, J., Turner, D., Kruger, A., Johnson, P., Gambell, J., & Linch, D. (2013). Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. The Lancet, 381(9880), 1817-1826.
- Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., Evdemon-Hogan, M., Conejo-Garcia, J. R., Zhang, L., Burow, M., Zhu, Y., Wei, S., Kryczek, I., Daniel, B., Gordon, A., Myers, L., Lackner, A., Disis, M. L., Knutson, K. L., . . . Zou, W. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine, 10(9), 942-949.
- Davis, R. E., Ngo, V. N., Lenz, G., Tolar, P., Young, R. M., Romesser, P. B., Kohlhammer, H., Lamy, L., Zhao, H., Yang, Y., Xu, W., Shaffer, A. L., Wright, G., Xiao, W., Powell, J., Jiang, J. K., Thomas, C. J., Rosenwald, A., Ott, G., . . . Staudt, L. M. (2010). Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature, 463(7277), 88-92.
- Deaglio, S., Dwyer, K. M., Gao, W., Friedman, D., Usheva, A., Erat, A., Chen, J. F., Enjyoji, K., Linden, J., Oukka, M., Kuchroo, V. K., Strom, T. B., & Robson, S. C. (2007). Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. Journal of Experimental Medicine, 204(6), 1257- 1265.
- Dedobbeleer, O., Stockis, J., van der Woning, B., Coulie, P. G., & Lucas, S. (2017). Cutting Edge: Active TGF-β1 Released from GARP/TGF-β1 Complexes on the Surface of Stimulated Human B Lymphocytes Increases Class-Switch Recombination and Production of IgA. The Journal of Immunology, 199(2), 391-396.
- Elkord, E., Alcantar-Orozco, E. M., Dovedi, S. J., Tran, D. Q., Hawkins, R. E., & Gilham, D. E. (2010). T regulatory cells in cancer: recent advances and therapeutic potential. Expert Opinion on Biological Therapy, 10(11), 1573-1586.
- Ernst, M., & Putoczki, T. (2014). IL-17 Cuts to the Chase in Colon Cancer. Immunity, 41(6), 880-882. doi:
- Facciabene, A., Peng, X., Hagemann, I. S., Balint, K., Barchetti, A., Wang, L. P., Gimotty, P. A., Gilks, C. B., Lal, P., Zhang, L., & Coukos, G. (2011). Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature, 475(7355), 226-230.
- Franzese, O., Kennedy, P. T. F., Gehring, A. J., Gotto, J., Williams, R., Maini, M. K., & Bertoletti, A. (2005). Modulation of the CD8 -T-Cell Response by CD4 CD25 Regulatory T Cells in Patients with Hepatitis B Virus Infection. Journal of Virology, 79(6), 3322-3328.
- Garcia-Hernandez, M. L., Hernandez-Pando, R., Gariglio, P., & Berumen, J. (2002). Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology, 105(2), 231-243.
- Gavin, M. A., Rasmussen, J. P., Fontenot, J. D., Vasta, V., Manganiello, V. C., Beavo, J. A., & Rudensky, A. Y. (2007). Foxp3- dependent programme of regulatory T- cell differentiation. Nature, 445(7129), 771-775.
- Ghiringhelli, F., MeÃŒÂNard, C., Terme, M., Flament, C., Taieb, J., Chaput, N., Puig, P. E., Novault, S., Escudier, B., Vivier, E., Lecesne, A., Robert, C., Blay, J. Y., Bernard, J., Caillat-Zucman, S., Freitas, A., Tursz, T., Wagner-Ballon, O., Capron, C., . . . Zitvogel, L. (2005). CD4 CD25 regulatory T cells inhibit natural killer cell functions in a transforming growth factor- β-dependent manner. Journal of Experimental Medicine, 202(8), 1075- 1085.
- Hemdan, N. Y. A. (2013). Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunology Letters, 149(1), 123- 133doi:
- Hu, Z., Luo, D., Wang, D., Ma, L., Zhao, Y., & Li, L. (2017). IL-17 Activates the IL-6/STAT3 Signal Pathway in the Proliferation of Hepatitis B Virus-Related Hepatocellular Carcinoma. Cellular Physiology and Biochemistry, 43(6), 2379-2390.
- Huber, S., Schramm, C., Lehr, H. A., Mann, A., Schmitt, S., Becker, C., Protschka, M., Galle, P. R., Neurath, M. F., & Blessing, M. (2004). Cutting Edge: TGF-β Signaling Is Required for the In Vivo Expansion and Immunosuppressive Capacity of Regulatory CD4 CD25 T Cells. The Journal of Immunology, 173(11), 6526- 6531.
- Hussain, S. P., Hofseth, L. J., & Harris, C. C. (2003). Radical causes of cancer. Nature Reviews Cancer, 3(4), 276-285.
- Ishida, T., Ishii, T., Inagaki, A., Yano, H., Komatsu, H., Iida, S., Inagaki, H., & Ueda, R. (2006). Specific Recruitment of CC Chemokine Receptor 4-Positive Regulatory T Cells in Hodgkin Lymphoma Fosters Immune Privilege. Cancer Research, 66(11), 5716-5722.
- Ivanov, I. I., McKenzie, B. S., Zhou, L., Tadokoro, C. E., Lepelley, A., Lafaille, J. J., . . . Littman, D. R. (2006). The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17 T Helper Cells. Cell, 126(6), 1121-1133. doi:
- Jin, P., Ren, H., Sun, W., Xin, W., Zhang, H., & Hao, J. (2014). Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Human Immunology, 75(1), 29- 33. doi:
- Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A., & Caton, A. J. (2001). Thymic selection of CD4 CD25 regulatory T cells induced by an agonist self-peptide. Nature Immunology, 2(4), 301-306.
- Kanzler, H., Küppers, R., Hansmann, M. L., & Rajewsky, K. (1996). Hodgkin and Reed- Sternberg cells in Hodgkin's disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. Journal of Experimental Medicine, 184(4), 1495- 1505.
- Karimi-Googheri, M., Daneshvar, H., Nosratabadi, R., Zare-Bidaki, M., Hassanshahi, G., Ebrahim, M., Arababadi, M. K., & Kennedy, D. (2013). Important roles played by TGF-β in hepatitis B infection. Journal of Medical Virology, 86(1), 102-108.
- Kobie, J. J., Shah, P. R., Yang, L., Rebhahn, J. A., Fowell, D. J., & Mosmann, T. R. (2006). T Regulatory and Primed Uncommitted CD4 T Cells Express CD73, Which Suppresses Effector CD4 T Cells by Converting 5′-Adenosine Monophosphate to Adenosine. The Journal of Immunology, 177(10), 6780- 6786.
- Küppers, R. (2008). The biology of Hodgkin's lymphoma. Nature Reviews Cancer, 9(1), 15-27.
- Küppers, R., Rajewsky, K., Zhao, M., Simons, G., Laumann, R., Fischer, R., & Hansmann, M. L. (1994). Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proceedings of the National Academy of Sciences, 91(23), 10962-10966.
- Küppers, R., Zhao, M., Hansmann, M. L., & Rajewsky, K. (1993). Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. 12(13), 4955- 4967. doi:
- Li, Q., Grover, A. C., Donald, E. J., Carr, A., Yu, J., Whitfield, J., Nelson, M., Takeshita, N., & Chang, A. E. (2005). Simultaneous Targeting of CD3 on T Cells and CD40 on B or Dendritic Cells Augments the Antitumor Reactivity of Tumor-Primed Lymph Node Cells. The Journal of Immunology, 175(3), 1424-1432.
- Liu, V. C., Wong, L. Y., Jang, T., Shah, A. H., Park, I., Yang, X., Zhang, Q., Lonning, S., Teicher, B. A., & Lee, C. (2007). Tumor Evasion of the Immune System by Converting CD4 CD25 T Cells into CD4 CD25 T Regulatory Cells: Role of Tumor-Derived TGF-β. The Journal of Immunology, 178(5), 2883-2892.
- Loder, B. F., Mutschler, B., Ray, R. J., Paige, C. J., Sideras, P., Torres, R., Lamers, M. C., & Carsetti, R. (1999). B Cell Development in the Spleen Takes Place in Discrete Steps and Is Determined by the Quality of B Cell Receptor-Derived Signals. Journal of Experimental Medicine, 190(1), 75-90.
- Lossos, I. S., Alizadeh, A. A., Eisen, M. B., Chan, W. C., Brown, P. O., Botstein, D., Staudt, L. M., & Levy, R. (2000). Ongoing immunoglobulin somatic mutation in germinal center B cell-like but not in activated B cell-like diffuse large cell lymphomas. Proceedings of the National Academy of Sciences, 97(18), 10209- 10213.
- Malek, T. R. (2008). The Biology of Interleukin- 2. Annual Review of Immunology, 26(1), 453-479.
- Mandapathil, M., Hilldorfer, B., Szczepanski, M. J., Czystowska, M., Szajnik, M., Ren, J., . . . Whiteside, T. L. (2010). Generation and Accumulation of Immunosuppressive Adenosine by Human CD4 CD25highFOXP3 Regulatory T Cells*. Journal of Biological Chemistry, 285(10), 7176-7186. doi:
- Marshall, E. A., Ng, K. W., Kung, S. H. Y., Conway, E. M., Martinez, V. D., Halvorsen, E. C., Rowbotham, D. A., Vucic, E. A., Plumb, A. W., Becker-Santos, D. D., Enfield, K. S. S., Kennett, J. Y., Bennewith, K. L., Lockwood, W. W., Lam, S., English, J. C., Abraham, N., & Lam, W. L. (2016). Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Molecular Cancer, 15(1).
- Meeker, T. C., Lowder, J., Maloney, D. G., Miller, R. A., Thielemans, K., Warnke, R., & Levy, R. (1985). A Clinical Trial of Anti- Idiotype Therapy for B Cell Malignancy. Blood, 65(6), 1349-1363. doi:
- Murai, M., Turovskaya, O., Kim, G., Madan, R., Karp, C. L., Cheroutre, H., & Kronenberg, M. (2009). Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature Immunology, 10(11), 1178-1184.
- Nurieva, R. I., Chung, Y., Hwang, D., Yang, X. O., Kang, H. S., Ma, L., . . . Dong, C. (2008). Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity, 29(1), 138-149. doi:
- Ohta, A., & Sitkovsky, M. (2014). Extracellular Adenosine-Mediated Modulation of Regulatory T Cells. Frontiers in Immunology, 5.
- Pan, B., Shen, J., Cao, J., Zhou, Y., Shang, L., Jin, S., Cao, S., Che, D., Liu, F., & Yu, Y. (2015). Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non- small-cell lung cancer. Scientific Reports, 5(1).
- Petanidis, S., Anestakis, D., Argyraki, M., Hadzopoulou-Cladaras, M., & Salifoglou, A. (2013). Differential Expression of IL-17, 22 and 23 in the Progression of Colorectal Cancer in Patients with K-ras Mutation: Ras Signal Inhibition and Crosstalk with GM-CSF and IFN-γ. PLoS ONE, 8(9), e73616.
- Pylayeva-Gupta, Y. (2016). Molecular Pathways: Interleukin-35 in Autoimmunity and Cancer. Clinical Cancer Research, 22(20), 4973-4978.
- Reth, M., & Nielsen, P. (2014). Chapter Four - Signaling Circuits in Early B-Cell Development. In F. W. Alt (Ed.), Advances in Immunology 122, 129-175, Academic Press.
- Rickert, R. C. (2013). New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nature Reviews Immunology, 13(8), 578-591.
- Romagnani, S. (1992). Type 1 T helper and type 2 T helper cells: Functions, regulation and role in protection and disease. International Journal of Clinical & Laboratory Research, 21(2-4), 152-158.
- Rosenwald, A., Wright, G., Chan, W. C., Connors, J. M., Campo, E., Fisher, R. I., Gascoyne, R. D., Muller-Hermelink, H. K., Smeland, E. B., Giltnane, J. M., Hurt, E. M., Zhao, H., Averett, L., Yang, L., Wilson, W. H., Jaffe, E. S., Simon, R., Klausner, R. D., Powell, J., . . . Staudt, L. M. (2002). The Use of Molecular Profiling to Predict Survival after Chemotherapy for Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine, 346(25), 1937-1947.
- Sawant, D. V., Yano, H., Chikina, M., Zhang, Q., Liao, M., Liu, C., Callahan, D. J., Sun, Z., Sun, T., Tabib, T., Pennathur, A., Corry, D. B., Luketich, J. D., Lafyatis, R., Chen, W., Poholek, A. C., Bruno, T. C., Workman, C. J., & Vignali, D. A. A. (2019). Adaptive plasticity of IL-10 and IL-35 Treg cells cooperatively promotes tumor T cell exhaustion. Nature Immunology, 20(6), 724-735.
- Seifert, M., Scholtysik, R., & Küppers, R. (2013). Origin and Pathogenesis of B Cell Lymphomas. In R. Küppers (Ed.), Lymphoma: Methods and Protocols (pp. 1-25). Totowa, NJ: Humana Press.
- Seo, N., Hayakawa, S., Takigawa, M., & Tokura, Y. (2001). Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4 T-regulatory cells and systemic collapse of antitumour immunity. Immunology, 103(4), 449-457.
- Seoane, J., & Gomis, R. R. (2017). TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harbor Perspectives in Biology, 9(12), a022277.
- Shaffer, A. L., Young, R. M., & Staudt, L. M. (2012). Pathogenesis of Human B Cell Lymphomas. Annual Review of Immunology, 30(1), 565-610.
- Shah, S., Divekar, A. A., Hilchey, S. P., Cho, H.- M., Newman, C. L., Shin, S.-U., . . . Rosenblatt, J. D. (2005). Increased rejection of primary tumors in mice lacking B cells: Inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. 117(4), 574-586. doi:
- Sitkovsky, M., Lukashev, D., Deaglio, S., Dwyer, K., Robson, S. C., & Ohta, A. (2008). Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. 153(S1), S457-S464. doi:
- Song, Y., & Yang, J. M. (2017). Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochemical and Biophysical Research Communications, 493(1), 1-8. doi:
- Sorrentino, R., Morello, S., Forte, G., Montinaro, A., Vita, G. D., Luciano, A., Palma, G., Arra, C., Maiolino, P., Adcock, I. M., & Pinto, A. (2011). B Cells Contribute to the Antitumor Activity of CpG- Oligodeoxynucleotide in a Mouse Model of Metastatic Lung Carcinoma. American Journal of Respiratory and Critical Care Medicine, 183(10), 1369-1379.
- Stockis, J., Fink, W., François, V., Connerotte, T., de Smet, C., Knoops, L., . . . Lucas, S. (2009). Comparison of stable human Treg and Th clones by transcriptional profiling. 39(3), 869-882. doi:
- Stoop, J. N., van der Molen, R. G., Kuipers, E. J., Kusters, J. G., & Janssen, H. L. A. (2007). Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology, 361(1), 141-148. doi:
- Strauss, L., Bergmann, C., Szczepanski, M., Gooding, W., Johnson, J. T., & Whiteside, T. L. (2007). A Unique Subset of CD4 CD25high Foxp3 T Cells Secreting Interleukin-10 and Transforming Growth Factor-β1 Mediates Suppression in the Tumor Microenvironment. Clinical Cancer Research, 13(15), 4345-4354.
- Su, Z. J., Yu, X. P., Guo, R. Y., Ming, D. S., Huang, L. Y., Su, M. L., Deng, Y., & Lin, Z. Z. (2013). Changes in the balance between Treg and Th17 cells in patients with chronic hepatitis B. Diagnostic Microbiology and Infectious Disease,76(4), 437-444.
- Thornton, A. M., Lu, J., Korty, P. E., Kim, Y. C., Martens, C., Sun, P. D., & Shevach, E. M. (2019). Helios and Helios Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. 49(3), 398-412. doi:
- Tilly, H., Gomes da Silva, M., Vitolo, U., Jack, A., Meignan, M., Lopez-Guillermo, A., . . . Ladetto, M. (2015). Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology, 26, v116-v125. doi:
- Trehanpati, N., & Vyas, A. K. (2017). Immune Regulation by T Regulatory Cells in Hepatitis B Virus-Related Inflammation and Cancer. Scandinavian Journal of Immunology, 85(3), 175-181.
- Trehanpati, N., Shrivastav, S., Shivakumar, B., Khosla, R., Bhardwaj, S., Chaturvedi, J., Sukriti, Kumar, B., Bose, S., Tripathi, D. M., Das, T., Sakhuja, P., Rastogi, A., Bhihari, C., Singh, S., Gupta, S., Kottilil, S., & Sarin, S. K. (2012). Analysis of Notch and TGF-β Signaling Expression in Different Stages of Disease Progression During Hepatitis B Virus Infection. Clinical and Translational Gastroenterology, 3(10), e23.
- Turnis, M. E., Sawant, D. V., Szymczak- Workman, A. L., Andrews, L. P., Delgoffe, G. M., Yano, H., . . . Vignali, D. A. A. (2016). Interleukin-35 Limits Anti-Tumor Immunity. Immunity, 44(2), 316-329. doi:
- Venkitaraman, A. R., Williams, G. T., Dariavach, P., & Neuberger, M. S. (1991). The B-cell antigen receptor of the five immunoglobulin classes. Nature, 352(6338), 777-781.
- Vignali, D. A. A., Collison, L. W., & Workman, C. J. (2008). How regulatory T cells work. Nature Reviews Immunology, 8(7), 523- 532.
- Voron, T., Marcheteau, E., Pernot, S., Colussi, O., Tartour, E., Taieb, J., & Terme, M. (2014). Control of the Immune Response by Pro- Angiogenic Factors. Frontiers in Oncology, 4.
- Wang, L., Yi, T., Kortylewski, M., Pardoll, D. M., Zeng, D., & Yu, H. (2009). IL-17 can promote tumor growth through an IL-6- Stat3 signaling pathway. Journal of Experimental Medicine, 206(7), 1457- 1464.
- Wang, S., Gao, X., Shen, G., Wang, W., Li, J., Zhao, J., Wei, Y. Q., & Edwards, C. K. (2016). Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Scientific Reports, 6(1).
- Young, R. M., Wu, T., Schmitz, R., Dawood, M., Xiao, W., Phelan, J. D., Xu, W., Menard, L., Meffre, E., Chan, W. C. C., Jaffe, E. S., Gascoyne, R. D., Campo, E., Rosenwald, A., Ott, G., Delabie, J., Rimsza, L. M., & Staudt, L. M. (2015). Survival of human lymphoma cells requires B-cell receptor engagement by self-antigens. Proceedings of the National Academy of Sciences, 112(44), 13447-13454.
- Yu, X., Guo, R., Ming, D., Su, M., Lin, C., Deng, Y., Lin, Z., & Su, Z. (2014). Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor- β1/interleukin-17 to be associated with the development of hepatitis B virus- associated liver cirrhosis. Journal of Gastroenterology and Hepatology, 29(5), 1065-1072.
- Zarek, P. E., Huang, C. T., Lutz, E. R., Kowalski, J., Horton, M. R., Linden, J., Drake, C. G., & Powell, J. D. (2008). A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood, 111(1), 251-259.
- Zhao, D. M., Thornton, A. M., DiPaolo, R. J., & Shevach, E. M. (2006). Activated CD4 CD25 T cells selectively kill B lymphocytes. Blood, 107(10), 3925-3932.
- Zheng, S. G., Wang, J., Wang, P., Gray, J. D., & Horwitz, D. A. (2007). IL-2 Is Essential for TGF-β to Convert Naive CD4 CD25 Cells to CD25 Foxp3 Regulatory T Cells and for Expansion of These Cells. The Journal of Immunology, 178(4), 2018- 2027.
- Zhu, X., & Zhu, J. (2020). CD4 T Helper Cell Subsets and Related Human Immunological Disorders. International Journal of Molecular Sciences, 21(21), 8011.
Cite this article
-
APA : Khan, R. M. A., Hassan, U. u., & Rehman, S. u. (2021). A Review on Immune Cells Mediated Tumor Progression. Global Drug Design & Development Review, VI(IV), 13-29. https://doi.org/10.31703/gdddr.2021(VI-IV).02
-
CHICAGO : Khan, Rana Muhammad Awais, Umair ul Hassan, and Shafiq ur Rehman. 2021. "A Review on Immune Cells Mediated Tumor Progression." Global Drug Design & Development Review, VI (IV): 13-29 doi: 10.31703/gdddr.2021(VI-IV).02
-
HARVARD : KHAN, R. M. A., HASSAN, U. U. & REHMAN, S. U. 2021. A Review on Immune Cells Mediated Tumor Progression. Global Drug Design & Development Review, VI, 13-29.
-
MHRA : Khan, Rana Muhammad Awais, Umair ul Hassan, and Shafiq ur Rehman. 2021. "A Review on Immune Cells Mediated Tumor Progression." Global Drug Design & Development Review, VI: 13-29
-
MLA : Khan, Rana Muhammad Awais, Umair ul Hassan, and Shafiq ur Rehman. "A Review on Immune Cells Mediated Tumor Progression." Global Drug Design & Development Review, VI.IV (2021): 13-29 Print.
-
OXFORD : Khan, Rana Muhammad Awais, Hassan, Umair ul, and Rehman, Shafiq ur (2021), "A Review on Immune Cells Mediated Tumor Progression", Global Drug Design & Development Review, VI (IV), 13-29
-
TURABIAN : Khan, Rana Muhammad Awais, Umair ul Hassan, and Shafiq ur Rehman. "A Review on Immune Cells Mediated Tumor Progression." Global Drug Design & Development Review VI, no. IV (2021): 13-29. https://doi.org/10.31703/gdddr.2021(VI-IV).02